Embark on an educational journey with our Acids and Bases Naming Worksheet! This comprehensive guide will equip you with the knowledge to confidently identify and name these fundamental chemical compounds. Prepare to unravel the secrets of acids and bases, their properties, and the IUPAC rules that govern their nomenclature.
Acids and bases play a pivotal role in various chemical reactions and everyday life. Understanding their characteristics and naming conventions is essential for students, researchers, and anyone interested in chemistry. This worksheet provides a clear and structured approach to mastering this topic.
Introduction
Understanding the nomenclature of acids and bases is crucial in chemistry as it enables us to identify and describe these substances accurately. Acids are chemical compounds that donate protons (H+ ions), while bases accept protons. There are various types of acids and bases, each with its own distinct properties and naming conventions.
Types of Acids
Acids can be classified based on their composition and the number of protons they can donate:
- Hydrohalic acids: Formed by the reaction of hydrogen with a halogen element (e.g., HCl, HBr, HI).
- Oxyacids: Contain hydrogen, oxygen, and another element (e.g., H2SO4, HNO3, H3PO4).
- Polyprotic acids: Can donate multiple protons (e.g., H2SO4, H3PO4).
Types of Bases
Bases can be classified based on their composition and the number of protons they can accept:
- Hydroxides: Compounds containing the hydroxide ion (OH-) (e.g., NaOH, KOH, Ca(OH)2).
- Amides: Derivatives of ammonia (NH3) in which one or more hydrogen atoms are replaced by an organic group (e.g., NH2-, NH-).
- Carbonates: Salts of carbonic acid (H2CO3) (e.g., Na2CO3, CaCO3).
Acids
Acids are chemical substances that share certain characteristic properties. They are typically sour in taste, corrosive to some materials, and react with metals to produce hydrogen gas. Acids also turn blue litmus paper red and have a pH below 7.
Identification of Acids
There are several methods to identify acids:
- pH Test:Acids have a pH below 7. pH is a measure of acidity or alkalinity on a scale of 0 to 14, with 7 being neutral. Values below 7 indicate acidity.
- Reaction with Metals:Acids react with certain metals, such as zinc or magnesium, to produce hydrogen gas. This reaction can be observed as bubbles forming on the metal surface.
- Litmus Paper Test:Acids turn blue litmus paper red. Litmus paper is a type of indicator paper that changes color in the presence of acids or bases.
Common Acids
Some common acids include:
- Hydrochloric acid (HCl):A strong acid used in various industrial processes, such as metal pickling and food processing.
- Sulfuric acid (H2SO 4): A highly corrosive acid used in fertilizers, batteries, and petroleum refining.
- Nitric acid (HNO3): A strong acid used in explosives, fertilizers, and dyes.
- Acetic acid (CH3COOH): A weak acid found in vinegar and used as a preservative.
- Citric acid (C6H 8O 7): A weak acid found in citrus fruits and used as a food additive and flavoring agent.
Bases: Acids And Bases Naming Worksheet
Bases are substances that can donate electrons or accept protons. They are typically characterized by a bitter taste, slippery feel, and ability to turn red litmus paper blue.
Bases can be identified using various methods, including:
- pH: Bases have a pH greater than 7.
- Reaction with acids: Bases react with acids to form salts and water. This reaction is known as neutralization.
Common Bases
Some common bases include:
- Sodium hydroxide (NaOH): A strong base used in various industrial and household applications, such as soap making and drain cleaning.
- Potassium hydroxide (KOH): Another strong base with similar uses to sodium hydroxide.
- Calcium hydroxide (Ca(OH)2): A weak base used in construction materials, such as mortar and plaster.
- Ammonia (NH3): A weak base with a characteristic pungent odor, used in household cleaning products and fertilizers.
Naming Acids and Bases
The International Union of Pure and Applied Chemistry (IUPAC) has established specific rules for naming acids and bases to ensure consistency and clarity in chemical nomenclature.
IUPAC Rules for Naming Acids
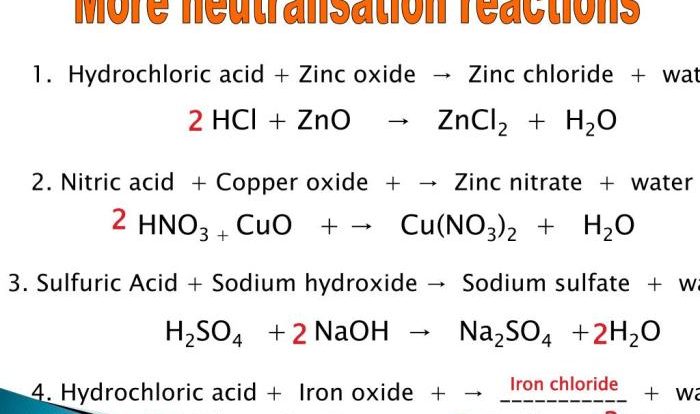
- Binary Acids:Contain hydrogen and one other element (e.g., HCl). Name the element with its root name, followed by “ic” and the word “acid.” (e.g., HCl is hydrochloric acid).
- Oxyacids:Contain hydrogen, oxygen, and another element. Name the element with its root name, followed by “ous” if it has a lower oxidation state and “ic” if it has a higher oxidation state. Then add the word “acid” (e.g., HNO 2is nitrous acid; HNO 3is nitric acid).
- Polyprotic Acids:Acids that can donate multiple protons. Use the prefixes “di,” “tri,” “tetra,” etc., before the acid name to indicate the number of protons (e.g., H 2SO 4is sulfuric acid; H 3PO 4is phosphoric acid).
IUPAC Rules for Naming Bases
- Hydroxides:Bases that contain the hydroxide ion (OH –). Name the metal or ammonium ion followed by “hydroxide” (e.g., NaOH is sodium hydroxide; NH 4OH is ammonium hydroxide).
- Oxides:Bases that contain oxygen and a metal. Name the metal followed by “oxide” (e.g., CaO is calcium oxide; Na 2O is sodium oxide).
- Ammonia and Amines:Weak bases that contain nitrogen. Ammonia (NH 3) is named as is. Amines are named by adding the suffix “-amine” to the name of the hydrocarbon group (e.g., CH 3NH 2is methylamine).
Practice Exercises
To enhance your understanding of acid and base naming, let’s delve into a series of practice exercises. These exercises are designed to provide a comprehensive assessment of your skills, ranging from basic to more challenging scenarios.
Each exercise consists of a chemical formula or name. Your task is to correctly identify and name the acid or base, as appropriate. Remember to apply the naming rules discussed earlier.
Easy Exercises, Acids and bases naming worksheet
- HCl
- NaOH
- H2SO4
- Ca(OH)2
Medium Exercises
- HNO3
- Al(OH)3
- H3PO4
- Fe(OH)2
Difficult Exercises
- H2CO3 (carbonic acid)
- CH3COOH (acetic acid)
- H3AsO4 (arsenic acid)
- NH4OH (ammonium hydroxide)
Answer Key
The answer key will be provided separately to allow you to self-assess your progress and identify areas for improvement.
Conclusion
In this worksheet, we have explored the fundamentals of naming acids and bases. Understanding this topic is crucial for comprehending various chemical reactions and processes.
Acids and bases play vital roles in numerous applications, ranging from industrial processes to biological systems. By mastering the ability to name these compounds accurately, you can effectively communicate chemical information and engage in scientific discussions.
Further Resources
- Chemistry LibreTexts: Naming Acids and Bases
- Khan Academy: Naming Acids and Bases
- Royal Society of Chemistry: Acids and Bases
Helpful Answers
What is the purpose of naming acids and bases?
Naming acids and bases allows us to identify and communicate about these compounds clearly and systematically.
How do I identify an acid?
Acids can be identified by their sour taste, ability to react with metals, and pH below 7.
What are the different types of acids?
There are various types of acids, including hydrochloric acid, sulfuric acid, and nitric acid.