Balancing chemical equations gizmos answer key – Delving into the realm of balancing chemical equations, this article unveils the intricacies of this fundamental concept in chemistry. As we explore the significance of balancing equations and delve into the Gizmos simulation, a comprehensive answer key will illuminate the path, empowering you to navigate this essential skill with confidence.
Unveiling the methods for balancing chemical equations, we will unravel the half-reaction and oxidation number approaches. Step-by-step instructions will guide you through the process, ensuring a thorough understanding of these techniques.
Balancing Chemical Equations
Balancing chemical equations involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation. This is important for ensuring the accuracy and validity of chemical reactions.
Methods for Balancing Chemical Equations: Balancing Chemical Equations Gizmos Answer Key
There are two main methods for balancing chemical equations: the half-reaction method and the oxidation number method. The half-reaction method involves dividing the overall reaction into two half-reactions, one for oxidation and one for reduction. The oxidation number method involves assigning oxidation numbers to each atom in the reaction and then adjusting the coefficients to balance the total oxidation numbers on both sides of the equation.
Half-Reaction Method, Balancing chemical equations gizmos answer key
- Identify the oxidation and reduction half-reactions.
- Balance each half-reaction in terms of mass and charge.
- Multiply the half-reactions by appropriate factors to make the number of electrons lost equal to the number of electrons gained.
- Add the two half-reactions together to obtain the balanced overall equation.
Oxidation Number Method
- Assign oxidation numbers to each atom in the reaction.
- Identify the atoms that are oxidized and reduced.
- Adjust the coefficients in front of the chemical formulas to balance the total oxidation numbers on both sides of the equation.
Gizmos Simulation
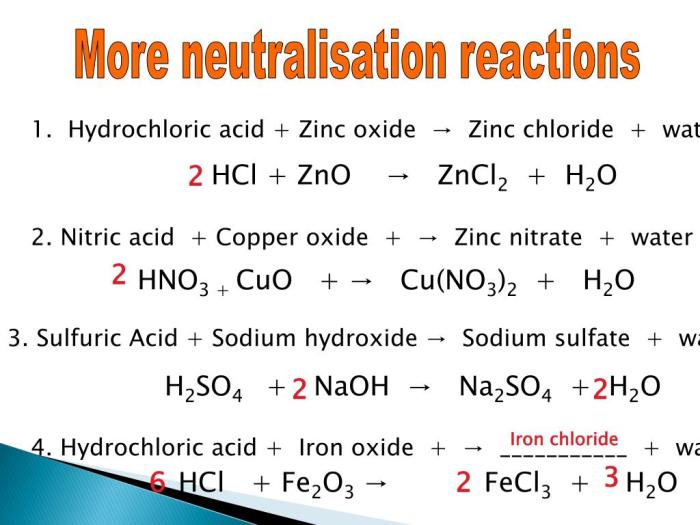
The Gizmos simulation for balancing chemical equations provides a virtual environment for students to practice balancing equations. The simulation allows students to input unbalanced equations and then provides step-by-step guidance on how to balance them using either the half-reaction method or the oxidation number method.
The Gizmos simulation can be used to:
- Practice balancing equations using both methods.
- Visualize the changes in oxidation numbers during a reaction.
- Identify common errors made when balancing equations.
Applications of Balancing Chemical Equations
Balancing chemical equations is essential for:
- Calculating stoichiometry (the quantitative relationships between reactants and products).
- Predicting the products of a chemical reaction.
- Understanding the mechanisms of chemical reactions.
Question Bank
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. This adherence to the law of conservation of mass is crucial for accurate stoichiometric calculations and a comprehensive understanding of chemical reactions.
How does the Gizmos simulation assist in balancing chemical equations?
The Gizmos simulation provides an interactive platform to practice balancing equations. It offers visual representations of chemical reactions, allowing you to manipulate coefficients and observe the changes in real-time. This hands-on approach reinforces the concepts and deepens your understanding.
Can I access the Gizmos answer key separately?
Yes, the Gizmos answer key is available as a separate resource. It provides detailed explanations for each question in the simulation, ensuring a thorough understanding of the balancing process and the underlying principles.