Embark on a journey of chemical discovery with our meticulously crafted Classifying and Balancing Chemical Reactions Worksheet. This interactive tool empowers you to delve into the fascinating world of chemical reactions, equipping you with the knowledge and skills to classify and balance these fundamental processes.
As you navigate through this worksheet, you will unravel the intricate mechanisms behind chemical transformations, gaining a deeper understanding of the interplay between reactants and products. Prepare to be captivated by the elegance and precision of chemical equations as you master the art of balancing them, ensuring that the conservation of mass is upheld.
Introduction
Chemical reactions are the processes in which one or more substances, the reactants, are transformed into one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Classifying and balancing chemical reactions are important for understanding and predicting the outcome of chemical processes. Classifying reactions helps us to organize and understand the vast number of reactions that can occur, while balancing reactions ensures that the number of atoms of each element is the same on both sides of the reaction equation.
Classifying Chemical Reactions: Classifying And Balancing Chemical Reactions Worksheet
Chemical reactions can be classified in a number of ways, depending on the criteria used. Some common classification methods include:
- By the type of change that occurs (e.g., combination, decomposition, single displacement, double displacement, combustion)
- By the energy change that occurs (e.g., exothermic, endothermic)
- By the rate at which the reaction occurs (e.g., fast, slow)
- By the state of the reactants and products (e.g., homogeneous, heterogeneous)
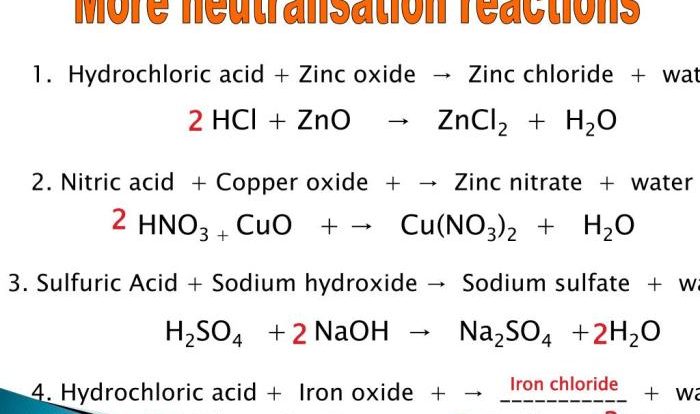
Balancing Chemical Reactions
Balancing chemical reactions involves adjusting the stoichiometric coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation. This is important because the law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction.
To balance a chemical reaction, the following steps can be followed:
- Write the unbalanced equation for the reaction.
- Identify the element that is unbalanced.
- Adjust the stoichiometric coefficient in front of the chemical formula for the compound containing the unbalanced element.
- Repeat steps 2 and 3 until all elements are balanced.
Applications of Classifying and Balancing Chemical Reactions
Classifying and balancing chemical reactions have a wide range of applications in various fields, including:
- Chemistry: Understanding and predicting the outcome of chemical reactions
- Chemical engineering: Designing and optimizing chemical processes
- Environmental science: Assessing and mitigating the environmental impact of chemical reactions
- Medicine: Developing and testing new drugs and therapies
- Materials science: Designing and developing new materials with specific properties
User Queries
What is the purpose of classifying chemical reactions?
Classifying chemical reactions helps us understand the different types of changes that can occur during a reaction, predict the products formed, and determine the stoichiometry of the reaction.
Why is it important to balance chemical equations?
Balancing chemical equations ensures that the law of conservation of mass is upheld, meaning that the total mass of the reactants is equal to the total mass of the products.