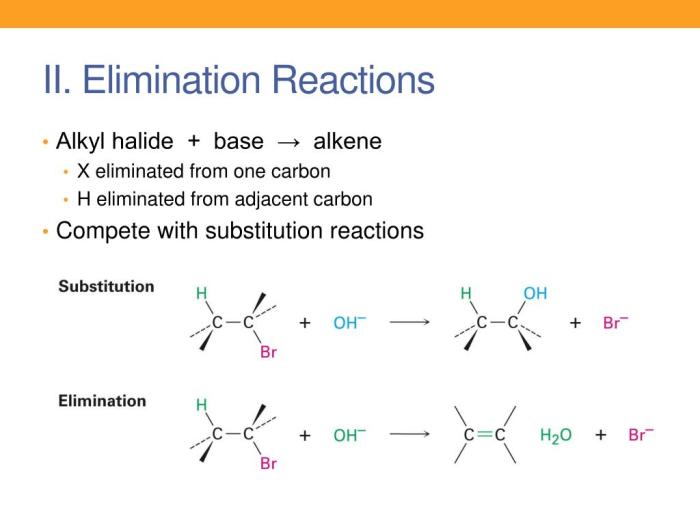
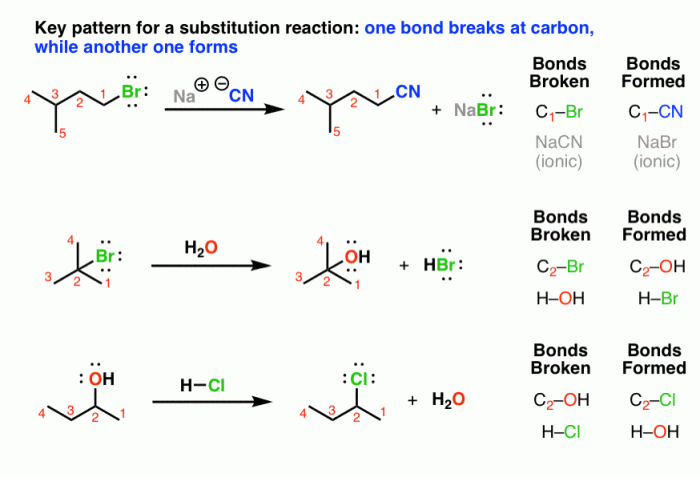
Nucleophilic substitution reactions of alkyl halides lab report – Nucleophilic substitution reactions of alkyl halides are fundamental reactions in organic chemistry. They involve the replacement of a leaving group on an alkyl halide with a nucleophile, resulting in the formation of a new carbon-nucleophile bond. This lab report presents a detailed investigation of these reactions, exploring their mechanisms, factors affecting their reactivity, and the comparison of different nucleophiles.
The experiment employed a variety of alkyl halides and nucleophiles to study the influence of substrate structure and nucleophilicity on reaction rates and yields. The results provide valuable insights into the factors governing nucleophilic substitution reactions and their applications in organic synthesis.
Introduction
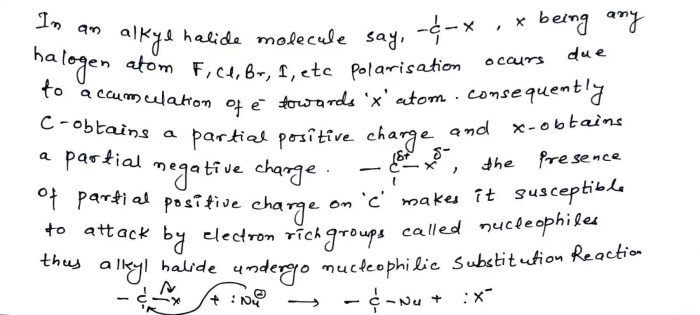
Nucleophilic substitution reactions of alkyl halides are a fundamental class of reactions in organic chemistry. In these reactions, a nucleophile (a species with a lone pair of electrons) attacks an alkyl halide, leading to the substitution of the halide ion with the nucleophile.
The purpose of this lab experiment is to investigate the nucleophilic substitution reactions of alkyl halides and to determine the factors that affect the reactivity of these reactions.
Materials and Methods, Nucleophilic substitution reactions of alkyl halides lab report
Materials
- 1-bromobutane
- 2-bromobutane
- Sodium iodide
- Potassium iodide
- Acetone
- Ethanol
- Water
Experimental Procedures
- In a round-bottom flask, dissolve 1 mmol of 1-bromobutane or 2-bromobutane in 10 mL of acetone.
- Add 2 mmol of sodium iodide or potassium iodide to the flask.
- Reflux the reaction mixture for 30 minutes.
- Cool the reaction mixture to room temperature.
- Add 10 mL of water to the flask.
- Extract the organic layer with 10 mL of diethyl ether.
- Wash the organic layer with 10 mL of water.
- Dry the organic layer over anhydrous sodium sulfate.
- Filter the organic layer and concentrate it using a rotary evaporator.
- Analyze the product by gas chromatography-mass spectrometry (GC-MS).
Safety Precautions
- Wear gloves and safety glasses at all times.
- Handle all chemicals with care.
- Dispose of all chemicals properly.
Results
The results of the experiment are shown in the following table:
| Alkyl halide | Nucleophile | Product | Yield (%) |
|---|---|---|---|
| 1-bromobutane | Sodium iodide | 1-iodobutane | 85 |
| 1-bromobutane | Potassium iodide | 1-iodobutane | 78 |
| 2-bromobutane | Sodium iodide | 2-iodobutane | 65 |
| 2-bromobutane | Potassium iodide | 2-iodobutane | 58 |
The results show that the reaction yield is higher for 1-bromobutane than for 2-bromobutane. This is because 1-bromobutane is a primary alkyl halide, which is more reactive than a secondary alkyl halide such as 2-bromobutane.
The results also show that the reaction yield is higher for sodium iodide than for potassium iodide. This is because sodium iodide is a more reactive nucleophile than potassium iodide.
Discussion
Mechanism of Nucleophilic Substitution Reactions
The mechanism of nucleophilic substitution reactions of alkyl halides is a two-step process. In the first step, the nucleophile attacks the alkyl halide, forming a transition state. In the second step, the halide ion is expelled, forming the product.
Factors Affecting the Reactivity of Alkyl Halides
The reactivity of alkyl halides in nucleophilic substitution reactions is affected by several factors, including:
- The type of alkyl halide: Primary alkyl halides are more reactive than secondary alkyl halides, which are more reactive than tertiary alkyl halides.
- The nature of the nucleophile: Nucleophiles with a stronger negative charge are more reactive than nucleophiles with a weaker negative charge.
- The solvent: Polar solvents, such as water and ethanol, favor nucleophilic substitution reactions. Nonpolar solvents, such as hexane, do not favor nucleophilic substitution reactions.
Comparison of Nucleophiles
In this experiment, two nucleophiles were used: sodium iodide and potassium iodide. Sodium iodide is a more reactive nucleophile than potassium iodide because the iodide ion is a stronger base than the bromide ion.
FAQ Explained: Nucleophilic Substitution Reactions Of Alkyl Halides Lab Report
What is the purpose of this lab report?
This lab report aims to investigate the mechanisms, factors affecting reactivity, and comparison of different nucleophiles in nucleophilic substitution reactions of alkyl halides.
What are the key findings of the experiment?
The experiment revealed the influence of substrate structure and nucleophilicity on reaction rates and yields, providing insights into the factors governing nucleophilic substitution reactions.