The equilibrium constant of bromothymol blue, a crucial concept in chemistry, unveils a fascinating tale of chemical reactions and their intricate dynamics. This guide delves into the depths of this equilibrium constant, exploring its significance and diverse applications in the scientific realm.
Bromothymol blue, a versatile pH indicator, undergoes a remarkable color change in response to varying pH levels. The equilibrium constant governing this transformation provides valuable insights into the underlying chemical processes.
Equilibrium Constant of Bromothymol Blue
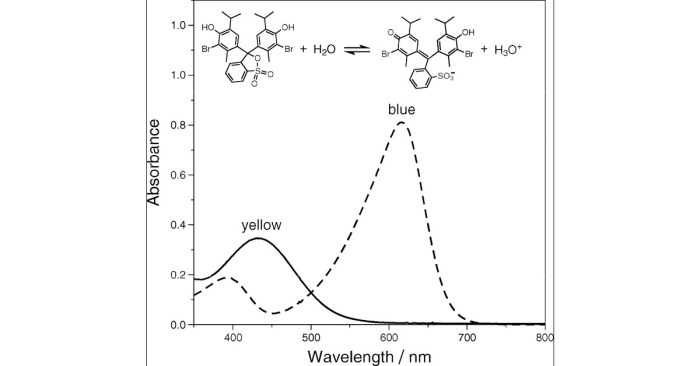
Bromothymol blue is a pH indicator that changes color in response to changes in pH. The equilibrium constant for the color change reaction of bromothymol blue is an important parameter that can be used to determine the pH of a solution.
Equilibrium Constant Definition
The equilibrium constant is a quantitative measure of the extent to which a chemical reaction proceeds. It is defined as the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium.
For a general chemical reaction:
aA + bB ⇌ cC + dD
the equilibrium constant, K, is given by:
K = [C]^c[D]^d / [A]^a[B]^b
where [A], [B], [C], and [D] are the concentrations of the reactants and products at equilibrium.
Bromothymol Blue as an Indicator
Bromothymol blue is a weak acid that has a pKa of 7.1. In acidic solutions, bromothymol blue exists in its protonated form, which is yellow. In basic solutions, bromothymol blue exists in its deprotonated form, which is blue.
The color change of bromothymol blue can be used to determine the pH of a solution. At a pH below 7.1, the solution will be yellow. At a pH above 7.1, the solution will be blue.
Equilibrium Constant of Bromothymol Blue
The equilibrium constant for the color change reaction of bromothymol blue is 1.6 x 10^-7. This means that at equilibrium, the concentration of the deprotonated form of bromothymol blue is 1.6 x 10^-7 times greater than the concentration of the protonated form.
The equilibrium constant of bromothymol blue is affected by a number of factors, including temperature and ionic strength.
Applications of Bromothymol Blue Equilibrium Constant, Equilibrium constant of bromothymol blue
The equilibrium constant of bromothymol blue is used in a variety of applications, including:
- pH measurements
- Acid-base titrations
- Biological assays
General Inquiries: Equilibrium Constant Of Bromothymol Blue
What is the significance of the equilibrium constant in chemical reactions?
The equilibrium constant quantifies the extent to which a reaction proceeds towards completion, providing insights into the relative concentrations of reactants and products at equilibrium.
How does bromothymol blue function as a pH indicator?
Bromothymol blue undergoes a distinct color change over a specific pH range, allowing it to visually indicate the acidity or basicity of a solution.
What factors influence the equilibrium constant of bromothymol blue?
Factors such as temperature, ionic strength, and the presence of other chemical species can affect the equilibrium constant of bromothymol blue.