Intro to stoichiometry moles to moles questions answer key – Embark on a comprehensive exploration of stoichiometry with our meticulously crafted guide, “Intro to Stoichiometry: Moles to Moles Questions and Answer Key.” This resource delves into the intricacies of chemical reactions, providing a solid foundation for understanding the quantitative relationships between reactants and products.
Prepare to unravel the mysteries of stoichiometry and master the art of mole-to-mole conversions.
Delving deeper into the realm of stoichiometry, we will unravel the concepts of molar mass, limiting reactants, theoretical and actual yields, and percent yield. Each concept is meticulously explained, complemented by illustrative examples and thought-provoking questions. Whether you are a student seeking clarity or an educator searching for engaging materials, this guide is your trusted companion.
1. Stoichiometry Fundamentals
Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It helps us predict the amount of reactants and products involved in a given reaction, based on the chemical equation.
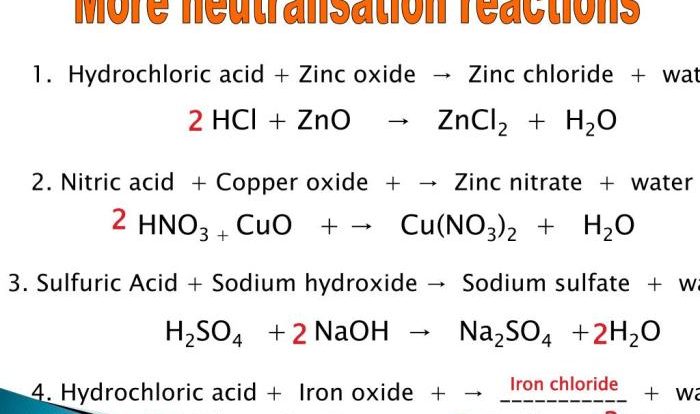
For example, in the combustion of methane, one molecule of methane (CH4) reacts with two molecules of oxygen (O2) to produce one molecule of carbon dioxide (CO2) and two molecules of water (H2O). The stoichiometric equation for this reaction is:
CH4 + 2O2 → CO2 + 2H2O
Stoichiometry plays a crucial role in chemistry, as it allows us to determine the limiting reactant, predict the theoretical yield of a reaction, and calculate the percent yield.
2. Moles and Molar Mass
The mole is the SI unit of amount of substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
Molar mass is the mass of one mole of a substance. It is calculated by adding the atomic masses of all the atoms in the chemical formula of the substance. For example, the molar mass of sodium chloride (NaCl) is 58.44 g/mol, which is the sum of the atomic masses of sodium (22.99 g/mol) and chlorine (35.45 g/mol).
Molar mass is a useful concept in stoichiometry, as it allows us to convert between the mass and the number of moles of a substance.
3. Mole-to-Mole Conversions
The mole-to-mole ratio in a chemical reaction is the ratio of the number of moles of reactants to the number of moles of products. This ratio is determined by the stoichiometric coefficients in the balanced chemical equation.
To perform mole-to-mole conversions, we use the following steps:
- Write the balanced chemical equation for the reaction.
- Identify the mole-to-mole ratio of the reactants and products.
- Multiply the number of moles of the known substance by the appropriate mole-to-mole ratio to find the number of moles of the unknown substance.
For example, if we want to find the number of moles of carbon dioxide produced when 2 moles of methane are burned, we can use the following steps:
- Write the balanced chemical equation: CH4 + 2O2 → CO2 + 2H2O
- Identify the mole-to-mole ratio: 1 mole of CH4 reacts with 1 mole of CO2
- Multiply the number of moles of CH4 by the mole-to-mole ratio: 2 moles of CH4 × 1 mole of CO2/1 mole of CH4 = 2 moles of CO2
4. Limiting Reactant and Excess Reactant
In a chemical reaction, the limiting reactant is the reactant that is completely consumed, while the excess reactant is the reactant that is left over after the reaction is complete.
To identify the limiting reactant, we can use the following steps:
- Calculate the number of moles of each reactant using their molar masses.
- Compare the number of moles of each reactant to the mole-to-mole ratio in the balanced chemical equation.
- The reactant that has the smallest mole-to-mole ratio is the limiting reactant.
5. Theoretical and Actual Yield
The theoretical yield of a reaction is the maximum amount of product that can be produced based on the stoichiometry of the reaction and the amount of reactants used.
The actual yield of a reaction is the amount of product that is actually obtained in the reaction.
The actual yield is often less than the theoretical yield due to factors such as side reactions, incomplete reactions, and losses during the isolation and purification of the product.
6. Percent Yield
The percent yield is a measure of the efficiency of a chemical reaction. It is calculated by dividing the actual yield by the theoretical yield and multiplying by 100%.
The percent yield can be used to evaluate the effectiveness of a reaction and to identify areas for improvement.
FAQ Corner: Intro To Stoichiometry Moles To Moles Questions Answer Key
What is the mole concept?
The mole is a fundamental unit in chemistry that represents a specific quantity of particles, typically atoms, molecules, or ions. It is defined as the amount of substance that contains exactly 6.02214076 × 10^23 entities.
How do I calculate the molar mass of a compound?
To calculate the molar mass of a compound, add the atomic masses of all the elements present in the compound, taking into account the number of atoms of each element.
What is the limiting reactant in a chemical reaction?
The limiting reactant is the reactant that is entirely consumed in a chemical reaction, thereby limiting the amount of product that can be formed.